Kimberly Petersen

Faculty Director, URSCO | Professor
International Honors College
Email Address: kspeters@uncg.edu
Phone: 336.334.4271
Biography
Dr. Kimberly Petersen is Director of the Undergraduate Research, Scholarship, and Creativity Office (URSCO) and Professor of Organic Chemistry in the Department of Chemistry and Biochemistry. A graduate of the University of Wisconsin-Madison and American University, she received her Ph.D. in Organic Chemistry from Johns Hopkins University. Before joining the faculty at UNC Greensboro she served as a Postdoctoral Fellow at the California Institute of Technology. Her Petersen research group, funded by the National Institutes of Health, is focused on solving important synthetic problems using basic principles of organic chemistry. In particular her lab is interested in the development of new methods for the asymmetric synthesis of biologically important molecules and in the design and synthesis of new drug targets. Dr. Petersen serves as a co-Principal Investigator on the MARC U-STAR/U-RISE Program at UNC Greensboro and is a recent recipient of the BRIDGES Academic Leadership Program.
Education
- B.S. Chemistry, University of Wisconsin-Madison, 2000
- M.S., Chemistry, American University, 2004
- Ph.D., Organic Chemistry, Johns Hopkins University, 2009
- Postdoctoral Fellow, California Institute of Technology, 2009-2011
Research
For more information visit: https://chem.uncg.edu/petersen/
The Petersen research group is focused on solving important synthetic problems using basic principles of organic chemistry. In particular we are interested in the development of new methods for the asymmetric synthesis of biologically important molecules and in the design and synthesis of new drug targets.
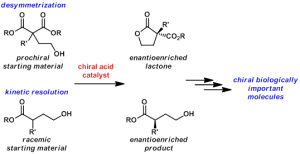
Many biologically important compounds contain a chiral center whose absolute configuration is critical for activity. Compounds found in nature that are identified with prospective utility in human health frequently exist as a complex mixture and are produced in such small quantities that they cannot be fully vetted for their potential. The exploration and future use of these precious materials often is contingent on the ability of a synthetic chemist to prepare them in an efficient and selective manner. Key to success of a laboratory synthesis of an enantioenriched complex molecule is the availability of simple enantioenriched building blocks. The ideal building block would have handles that are readily transformed into a variety of functionalities that can be incorporated into the biologically important molecule. A major focus of our research laboratory is the development of asymmetric methodologies for the synthesis of versatile enantioenriched building blocks.
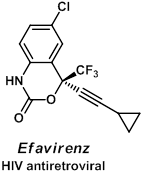
In recent years, fluorine containing molecules have become important in the pharmaceutical and agrochemical industries, and in particular, the trifluoromethyl group appears in many biologically active compounds such as the HIV antiretroviral drug Efavirenz. The increased lipophilicity and metabolic stability of the CF3 group often accounts for an improved activity profile. Unfortunately, the regio- and stereoselective incorporation of the trifluoromethyl group into organic molecules remains a challenge for chemists. Research toward the selective incorporation of this important functional group is another focus in our group.
Despite recent advances in drug therapies for diseases such as cancer and malaria there is an essential need for ever more innovative and effective small molecule therapeutics. The Petersen group is active in the design and synthesis of new scaffolds with antimalarial or anticancer properties, in particular novel peroxides and diphenylpropynones.
Representative Publications
- Petersen, K. S.; Stoltz, B. M. Palladium-catalyzed, asymmetric Baeyer–Villiger oxidation of prochiral cyclobutanones with PHOX ligands. Tetrahedron 2011, 67, 4352–4357.http://www.sciencedirect.com/science/article/pii/S0040402011005539
- Posner, G. H.; Chang, W.; Hess, L.; Woodard, L.; Sinishtaj, S.; Usera, A. R.; Maio, W.; Rosenthal, A. S.; Kalinda, A. S.; Petersen, K. S.; Stohler, R.; Chollet, J.; Santo-Tomas, J.; Snyder, C.; Rottmann, M.; Wittlin, S.; Brun, R.; Shapiro, T. A. Malaria-infected mice are cured by oral administration of new artemisinin derivatives. J. Med. Chem. 2008, 51, 1035–1042. http://pubs.acs.org/doi/abs/10.1021/jm701168h
- Petersen, K. S.; Posner, G. H. Asymmetric, organocatalytic, 3-step synthesis of γ-hydroxy-(E)-α,β-unsaturated sulfones and esters. Org. Lett. 2008, 10, 4685–4687. http://pubs.acs.org/doi/abs/10.1021/ol8020513
- Posner, G. H.; Suh, B. C.; Petersen, K. S.; Dolan, P.; Kensler, T. W.; Koh, J. T.; Peleg, S. Difluoromethyl analogs of the natural hormone 1α,25-dihydroxyvitamin D3: design, synthesis, and preliminary biological evaluation. J. Steroid Biochem. Mol. Biol. 2007, 103, 213–221. http://www.sciencedirect.com/science/article/pii/S0960076006003724
- Petersen, K. S.; Dolan, P. M.; Kensler, T. W.; Peleg, S.; Posner, G. H. Low-calcemic, highly antiproliferative, 23-oxa ether analogs of the natural hormone 1α,25-dihydroxyvitamin D3: design, synthesis, and preliminary biological evaluation. J. Med. Chem. 2007, 50, 5824–5832. http://pubs.acs.org/doi/abs/10.1021/jm070882d
- Peleg, S.; Petersen, K. S.; Suh, B. C.; Dolan, P.; Agoston, E. S.; Kensler, T. W.; Posner, G. H. Low-calcemic, highly antiproliferative, 1-difluoromethyl hybrid analogs of the natural hormone 1α,25-dihydroxy D3: design, synthesis, and preliminary biological evaluation. J. Med. Chem. 2006, 49, 7513–7517.http://pubs.acs.org/doi/abs/10.1021/jm0609925
- Thibout, E.; Arnault, I.; Auger, J.; Petersen, K. S.; Oliver, J. E. Characterization of a behaviorally active, gender-specific volatile compound from the male asparagus fly. J. Chem. Ecol. 2005, 31, 893–909.http://www.springerlink.com/content/m8256035522rp387/
- Copper, L. D.; Doss, R. P.; Price, R.; Peterson, K.; Oliver, J. E. Application of Bruchin B to pea pods results in the up-regulation of CYP93C18, a putative isoflavone synthase gene, and an increase in the level of pisatin, an isoflavone phytoalexin. J. Exp. Bot. 2005, 56, 1229–1237. http://jxb.oxfordjournals.org/content/56/414/1229.abstract